A Case-Series Observation of Sweat Rate Variability in Endurance-Trained Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Deurenberg, P.; Wang, W.; Pietrobelli, A.; Baumgartner, R.N.; Heymsfield, S.B. Hydration of fat-free body mass: Review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 1999, 69, 833–841. [Google Scholar] [CrossRef]
- Shibasaki, M.; Wilson, T.E.; Crandall, C.G. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J. Appl. Physiol. 2006, 100, 1692–1701. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C.; Casa, D.J.; Ganio, M.S.; McDermott, B.P.; Yamamoto, L.M.; Lopez, R.M.; Emmanuel, H. The American football uniform: Uncompensable heat stress and hyperthermic exhaustion. J. Athl. Train 2010, 45, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Osterberg, K.L.; Horswill, C.A.; Baker, L.B. Pregame urine specific gravity and fluid intake by National Basketball Association players during competition. J. Athl. Train 2009, 44, 53–57. [Google Scholar] [CrossRef]
- Volpe, S.L.; Poule, K.A.; Bland, E.G. Estimation of prepractice hydration status of National Collegiate Athletic Association Division I athletes. J. Athl. Train 2009, 44, 624–629. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Casa, D.J.; Yeargin, S.W.; Ganio, M.S.; Lopez, R.M.; Mooradian, E.A. Hydration status, sweat rates, and rehydration education of youth football campers. J. Sport Rehabil. 2009, 18, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alonso, J.; Mora-Rodriguez, R.; Below, P.R.; Coyle, E.F. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. J. Appl. Physiol 1995, 79, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National Athletic Trainers’ Association position statement: Fluid replacement for the physically active. J. Athl. Train 2017, 52, 877–895. [Google Scholar] [CrossRef]
- Nuccio, R.P.; Barnes, K.A.; Carter, J.M.; Baker, L.B. Fluid balance in team sport athletes and the effect of hypohydration on cognitive, technical, and physical performance. Sports Med. 2017, 47, 1951–1982. [Google Scholar] [CrossRef]
- Montain, S.J.; Sawka, M.N.; Latzka, W.A.; Valeri, C.R. Thermal and cardiovascular strain from hypohydration: Influence of exercise intensity. Int. J. Sports Med. 1998, 19, 87–91. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W.; Montain, S.J.; Sawka, M.N. Mechanisms of aerobic performance impairment with heat stress and dehydration. J. Appl. Physiol. 2010, 109, 1989–1995. [Google Scholar] [CrossRef]
- Walsh, R.M.; Noakes, T.D.; Hawley, J.A.; Dennis, S.C. Impaired high-intensity cycling performance time at low levels of dehydration. Int. J. Sports Med. 1994, 15, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Bardis, C.N.; Kavouras, S.A.; Arnaoutis, G.; Panagiotakos, D.B.; Sidossis, L.S. Mild dehydration and cycling performance during 5-kilometer hill climbing. J. Athl. Train 2013, 48, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Casa, D.J.; Stearns, R.L.; Lopez, R.M.; Ganio, M.S.; McDermott, B.P.; Walker Yeargin, S.; Yamamoto, L.M.; Mazerolle, S.M.; Roti, M.W.; Armstrong, L.E.; et al. Influence of hydration on physiological function and performance during trail running in the heat. J. Athl. Train 2010, 45, 147–156. [Google Scholar] [CrossRef]
- Ebert, T.R.; Martin, D.T.; Bullock, N.; Mujika, I.; Quod, M.J.; Farthing, L.A.; Burke, L.M.; Withers, R.T. Influence of hydration status on thermoregulation and cycling hill climbing. Med. Sci. Sports Exerc. 2007, 39, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Carter, R., 3rd; Castellani, J.W.; Sawka, M.N. Hypohydration impairs endurance exercise performance in temperate but not cold air. J. Appl. Physiol. 2005, 99, 1972–1976. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Carter, R., 3rd; Sawka, M.N. Fluid balance and endurance exercise performance. Curr. Sports Med. Rep. 2003, 2, 202–208. [Google Scholar] [CrossRef]
- Schrier, R.W.; Fassett, R.G.; Ohara, M.; Martin, P.Y. Pathophysiology of renal fluid retention. Kidney Int. Suppl. 1998, 67, S127–S132. [Google Scholar] [CrossRef][Green Version]
- Bartsch, P.; Maggiorini, M.; Schobersberger, W.; Shaw, S.; Rascher, W.; Girard, J.; Weidmann, P.; Oelz, O. Enhanced exercise-induced rise of aldosterone and vasopressin preceding mountain sickness. J. Appl. Physiol. 1991, 71, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T. Exercise-Associated Hyponatremia. Front. Horm. Res. 2019, 52, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.E.; Kay, D.; Cannon, J. Glycerol hyperhydration fails to improve endurance performance and thermoregulation in humans in a warm humid environment. Pflugers Arch. 2003, 446, 455–462. [Google Scholar] [CrossRef]
- Sawka, M.N.; Montain, S.J.; Latzka, W.A. Hydration effects on thermoregulation and performance in the heat. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 679–690. [Google Scholar] [CrossRef]
- Maresh, C.M.; Bergeron, M.F.; Kenefick, R.W.; Castellani, J.W.; Hoffman, J.R.; Armstrong, L.E. Effect of overhydration on time-trial swim performance. J. Strength Cond. Res. 2001, 15, 514–518. [Google Scholar] [PubMed]
- Gigou, P.Y.; Dion, T.; Asselin, A.; Berrigan, F.; Goulet, E.D. Pre-exercise hyperhydration-induced bodyweight gain does not alter prolonged treadmill running time-trial performance in warm ambient conditions. Nutrients 2012, 4, 949–966. [Google Scholar] [CrossRef]
- Goulet, E.D.; Aubertin-Leheudre, M.; Plante, G.E.; Dionne, I.J. A meta-analysis of the effects of glycerol-induced hyperhydration on fluid retention and endurance performance. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 391–410. [Google Scholar] [CrossRef]
- Monteith, J.L. Latent heat of vaporization in thermal physiology. Nat. New Biol. 1972, 236, 96. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating rate and sweat sodium concentration in athletes: A review of methodology and intra/interindividual variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef]
- Baker, L.B.; Barnes, K.A.; Anderson, M.L.; Passe, D.H.; Stofan, J.R. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes. J. Sports Sci. 2016, 34, 358–368. [Google Scholar] [CrossRef]
- Gonzalez, R.R.; Cheuvront, S.N.; Ely, B.R.; Moran, D.S.; Hadid, A.; Endrusick, T.L.; Sawka, M.N. Sweat rate prediction equations for outdoor exercise with transient solar radiation. J. Appl. Physiol. 2012, 112, 1300–1310. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Haymes, E.M.; Sawka, M.N. Comparison of sweat loss estimates for women during prolonged high-intensity running. Med. Sci. Sports Exerc. 2002, 34, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Armstrong, L.E.; Coyle, E.F.; Mack, G.W.; Sawka, M.N.; Senay, L.C., Jr.; Sherman, W.M. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 1996, 28, i–vii. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H.; Kirven, J. Exercise-associated hyponatremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 151–161. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Baker, L.B.; Stofan, J.R.; Hamilton, A.A.; Horswill, C.A. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise. J. Appl. Physiol. 2009, 107, 887–895. [Google Scholar] [CrossRef]
- Hayden, G.; Milne, H.C.; Patterson, M.J.; Nimmo, M.A. The reproducibility of closed-pouch sweat collection and thermoregulatory responses to exercise-heat stress. Eur. J. Appl. Physiol. 2004, 91, 748–751. [Google Scholar] [CrossRef]
- Twerenbold, R.; Knechtle, B.; Kakebeeke, T.H.; Eser, P.; Muller, G.; von Arx, P.; Knecht, H. Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br. J. Sports Med. 2003, 37, 300–303. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-associated hyponatremia: 2017 update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the third international exercise-associated hyponatremia consensus development conference, Carlsbad, California, 2015. Clin. J. Sport Med. 2015, 25, 303–320. [Google Scholar] [CrossRef]
- Hubbard, R.W.; Sandick, B.L.; Matthew, W.T.; Francesconi, R.P.; Sampson, J.B.; Durkot, M.J.; Maller, O.; Engell, D.B. Voluntary dehydration and alliesthesia for water. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 868–873. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Filingeri, D.; Havenith, G. Human skin wetness perception: Psychophysical and neurophysiological bases. Temperature 2015, 2, 86–104. [Google Scholar] [CrossRef]
- McLellan, T.M.; Daanen, H.A.; Cheung, S.S. Encapsulated environment. Compr. Physiol. 2013, 3, 1363–1391. [Google Scholar] [CrossRef]
- Mathews, D.K.; Fox, E.L.; Tanzi, D. Physiological responses during exercise and recovery in a football uniform. J. Appl. Physiol. 1969, 26, 611–615. [Google Scholar] [CrossRef]
- Cheung, S.S. Advanced Environmental Exercise Physiology; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Nielsen, B.; Hales, J.R.; Strange, S.; Christensen, N.J.; Warberg, J.; Saltin, B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J. Physiol. 1993, 460, 467–485. [Google Scholar] [CrossRef]
- Nielsen, B.; Strange, S.; Christensen, N.J.; Warberg, J.; Saltin, B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch. 1997, 434, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rollo, I.; Randell, R.K.; Baker, L.; Leyes, J.Y.; Medina Leal, D.; Lizarraga, A.; Mesalles, J.; Jeukendrup, A.E.; James, L.J.; Carter, J.M. Fluid balance, sweat Na+ losses, and carbohydrate intake of Elite male soccer players in response to low and high training intensities in cool and hot environments. Nutrients 2021, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Fortney, S.M.; Wenger, C.B.; Bove, J.R.; Nadel, E.R. Effect of hyperosmolality on control of blood flow and sweating. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Latzka, W.A.; Sawka, M.N. Control of thermoregulatory sweating is altered by hydration level and exercise intensity. J. Appl. Physiol. 1995, 79, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Cheuvront, S.N.; Lukaski, H.C. Sweat mineral-element responses during 7 h of exercise-heat stress. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Bar-Or, O.; Calvert, R.; MacDougall, J.D. Sweat gland response to exercise in the heat among pre-, mid-, and late-pubertal boys. Med. Sci. Sports Exerc. 1992, 24, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Falk, B.; Bar-Or, O.; MacDougall, J.D. Thermoregulatory responses of pre-, mid-, and late-pubertal boys to exercise in dry heat. Med. Sci. Sports Exerc. 1992, 24, 688–694. [Google Scholar] [CrossRef]
- Lee, H.; Petrofsky, J.; Shah, N.; Awali, A.; Shah, K.; Alotaibi, M.; Yim, J. Higher sweating rate and skin blood flow during the luteal phase of the menstrual cycle. Tohoku J. Exp. Med. 2014, 234, 117–122. [Google Scholar] [CrossRef]
- Garcia, A.M.; Lacerda, M.G.; Fonseca, I.A.; Reis, F.M.; Rodrigues, L.O.; Silami-Garcia, E. Luteal phase of the menstrual cycle increases sweating rate during exercise. Braz. J. Med. Biol. Res. 2006, 39, 1255–1261. [Google Scholar] [CrossRef]
- Janse, D.E.J.X.A.; Thompson, M.W.; Chuter, V.H.; Silk, L.N.; Thom, J.M. Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med. Sci. Sports Exerc. 2012, 44, 2190–2198. [Google Scholar] [CrossRef]
- Luetkemeier, M.J.; Allen, D.R.; Huang, M.; Pizzey, F.K.; Parupia, I.M.; Wilson, T.E.; Davis, S.L. Skin tattooing impairs sweating during passive whole body heating. J. Appl. Physiol. 2020, 129, 1033–1038. [Google Scholar] [CrossRef]
- Luetkemeier, M.J.; Hanisko, J.M.; Aho, K.M. Skin tattoos alter sweat rate and Na+ concentration. Med. Sci. Sports Exerc. 2017, 49, 1432–1436. [Google Scholar] [CrossRef]
- Beliveau, J.; Perreault-Briere, M.; Jeker, D.; Deshayes, T.A.; Duran-Suarez, A.; Baker, L.B.; Goulet, E.D.B. Permanent tattooing has no impact on local sweat rate, sweat sodium concentration and skin temperature or prediction of whole-body sweat sodium concentration during moderate-intensity cycling in a warm environment. Eur. J. Appl. Physiol. 2020, 120, 1111–1122. [Google Scholar] [CrossRef]
- Rogers, E.; Irwin, C.; McCartney, D.; Cox, G.R.; Desbrow, B. Tattoos do not affect exercise-induced localised sweat rate or sodium concentration. J. Sci. Med. Sport 2019, 22, 1249–1253. [Google Scholar] [CrossRef]
- Pandolf, K.B.; Gange, R.W.; Latzka, W.A.; Blank, I.H.; Kraning, K.K., 2nd; Gonzalez, R.R. Human thermoregulatory responses during heat exposure after artificially induced sunburn. Am. J. Physiol. 1992, 262, R610–R616. [Google Scholar] [CrossRef] [PubMed]
- Fealey, R.D.; Low, P.A.; Thomas, J.E. Thermoregulatory Sweating Abnormalities in Diabetes Mellitus. Mayo Clin. Proc. 1989, 64, 617–628. [Google Scholar] [CrossRef]
- Price, M.J. Thermoregulation during exercise in individuals with spinal cord injuries. Sports Med. 2006, 36, 863–879. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Campbell, I.G. Thermoregulatory responses of paraplegic and able-bodied athletes at rest and during prolonged upper body exercise and passive recovery. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 552–560. [Google Scholar] [CrossRef]
- Cheshire, W.P.; Fealey, R.D. Drug-induced hyperhidrosis and hypohidrosis: Incidence, prevention and management. Drug Saf. 2008, 31, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Lang, J.A.; Kenney, W.L. Change in body mass accurately and reliably predicts change in body water after endurance exercise. Eur. J. Appl. Physiol. 2009, 105, 959–967. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M. Development of individual hydration strategies for athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 457–472. [Google Scholar] [CrossRef]
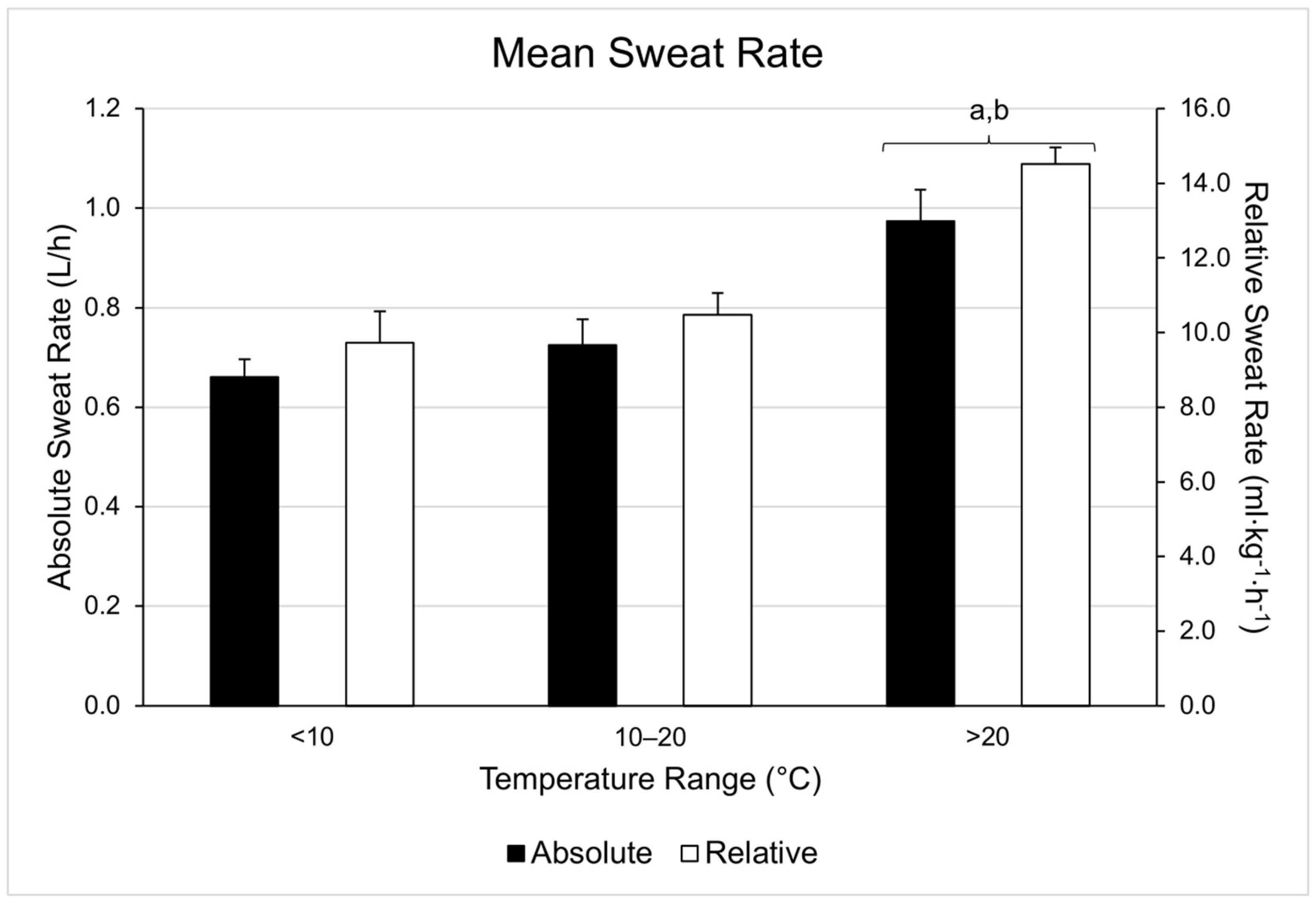
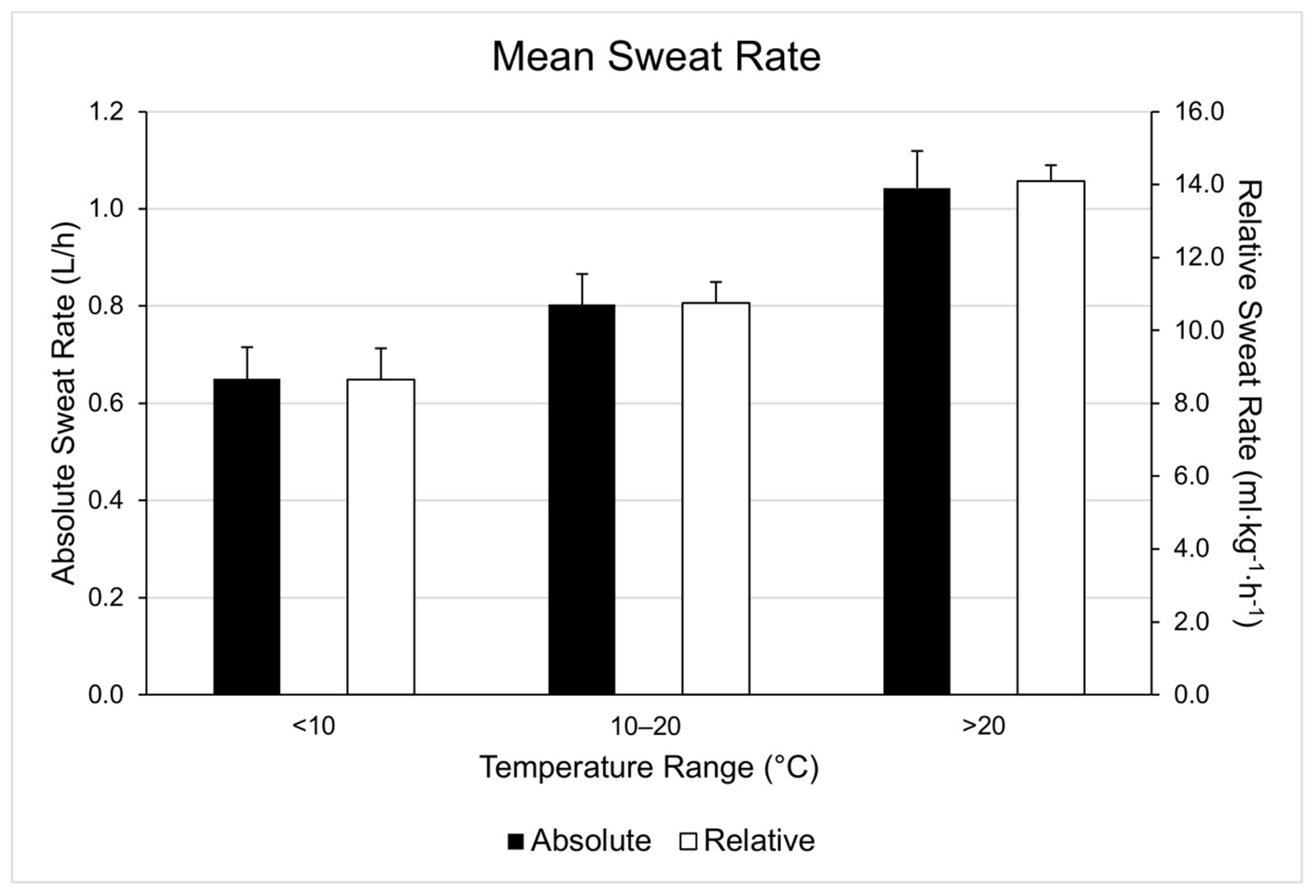
| Range | WBGT (°C) | Duration (Minutes) | Running Distance (Miles) | Running Pace (Min/Mile) | Cycling Distance (Miles) | Cycling Velocity (Miles/Hour) |
|---|---|---|---|---|---|---|
| <10 °C | 5.7 ± 3.2 | 42.67 ± 13.33 (n = 9) | 6.05 ± 4.67 (n = 8) | 8.40 ± 2.39 (n = 8) | 21.04 ± 0.03 (n = 1) | 17.43 ± 0.16 (n = 1) |
| 10–20 °C | 13.5 ± 2.2 | 40.63 ± 11.29 (n = 6) | 5.19 ± 1.82 (n = 6) | 8.07 ± 1.10 (n = 6) | ||
| >20 °C | 22.5 ± 1.4 | 43.29 ± 14.11 (n = 11) | 6.84 ± 5.60 (n = 10) | 7.62 ± 1.67 (n = 10) | 24.41 ± 4.53 (n = 1) | 19.34 ± 0.83 (n = 1) |
| 2A. | ||||||
| <10 °C | ||||||
| Subject | Low | High | Mean | Relative Mean | High-Low | Maximal Variation from Mean |
| (L/h) | (L/h) | (L/h) | (mL·kg−1·h−1) | (L/h) | (%) | |
| 1 | 0.84 | 0.90 | 0.87 | 13.5 | 0.06 | 3.4 |
| 2 | 0.43 | 0.52 | 0.48 | 8.5 | 0.09 | 9.4 |
| 3 | 0.41 | 0.56 | 0.48 | 7.2 | 0.15 | 15.5 |
| 4 | 0.65 | 0.95 | 0.84 | 8.7 | 0.30 | 17.8 |
| 5 | 0.97 | 1.00 | 0.99 | 9.6 | 0.03 | 1.5 |
| 8 | 0.54 | 0.61 | 0.57 | 9.1 | 0.07 | 6.2 |
| 9 | 0.57 | 0.75 | 0.66 | 10.2 | 0.18 | 13.7 |
| 11 | 0.39 | 0.52 | 0.45 | 5.2 | 0.13 | 14.4 |
| 12 | 0.67 | 0.90 | 0.78 | 12.6 | 0.23 | 14.8 |
| 2B. | ||||||
| 10–20 °C | ||||||
| Subject | Low | High | Mean | Relative Mean | High-Low | Maximal Variation from Mean |
| (L/h) | (L/h) | (L/h) | (mL·kg−1·h−1) | (L/h) | (%) | |
| 1 | 1.13 | 1.23 | 1.18 | 11.6 | 0.10 | 4.2 |
| 2 | 0.43 | 0.68 | 0.60 | 9.0 | 0.25 | 21.0 |
| 3 | 0.58 | 0.78 | 0.64 | 11.9 | 0.20 | 15.5 |
| 4 | 0.83 | 0.84 | 0.84 | 10.2 | 0.01 | 0.6 |
| 6 | 0.46 | 0.79 | 0.57 | 9.2 | 0.33 | 29.1 |
| 10 | 0.49 | 0.49 | 0.49 | 8.5 | 0.00 | 0.0 |
| 2C. | ||||||
| >20 °C | ||||||
| Subject | Low | High | Mean | Relative Mean | High-Low | Maximal Variationfrom Mean |
| (L/h) | (L/h) | (L/h) | (mL·kg−1·h−1) | (L/h) | (%) | |
| 1 | 1.32 | 1.35 | 1.33 | 13.44 | 0.03 | 1.2 |
| 2 | 0.81 | 1.10 | 0.90 | 13.9 | 0.29 | 16.1 |
| 3 | 0.75 | 0.96 | 0.85 | 16.1 | 0.21 | 12.3 |
| 4 | 1.16 | 1.16 | 1.16 | 13.0 | 0.00 | 0.0 |
| 5 | 1.62 | 1.70 | 1.67 | 23.0 | 0.08 | 2.4 |
| 6 | 0.63 | 0.81 | 0.72 | 12.5 | 0.18 | 12.5 |
| 7 | 0.76 | 0.91 | 0.82 | 13.8 | 0.15 | 9.2 |
| 8 | 0.64 | 0.74 | 0.69 | 11.9 | 0.10 | 7.2 |
| 9 | 1.01 | 1.22 | 1.11 | 17.6 | 0.21 | 9.4 |
| 10 | 0.58 | 0.59 | 0.59 | 10.4 | 0.01 | 0.9 |
| 13 | 0.79 | 0.81 | 0.80 | 11.6 | 0.02 | 0.1 |
| Subject | Temperature | Low | High | Mean |
|---|---|---|---|---|
| 1 | <10 °C | 0.84 L/h | 0.90 L/h | 0.87 L/h |
| 10–20 °C | 1.13 L/h | 1.23 L/h | 1.18 L/h | |
| >20 °C | 1.32 L/h | 1.35 L/h | 1.33 L/h | |
| 2 | <10 °C | 0.43 L/h | 0.52 L/h | 0.48 L/h |
| 10–20 °C | 0.43 L/h | 0.68 L/h | 0.60 L/h | |
| >20 °C | 0.81 L/h | 1.10 L/h | 0.90 L/h | |
| 3 | <10 °C | 0.41 L/h | 0.56 L/h | 0.48 L/h |
| 10–20 °C | 0.58 L/h | 0.78 L/h | 0.64 L/h | |
| >20 °C | 0.75 L/h | 0.96 L/h | 0.85 L/h | |
| 4 | <10 °C | 0.65 L/h | 0.95 L/h | 0.84 L/h |
| 10–20 °C | 0.83 L/h | 0.84 L/h | 0.84 L/h | |
| >20 °C | 1.16 L/h | 1.16 L/h | 1.16 L/h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.W.; Bello, M.L.; Price, F.G. A Case-Series Observation of Sweat Rate Variability in Endurance-Trained Athletes. Nutrients 2021, 13, 1807. https://doi.org/10.3390/nu13061807
Smith JW, Bello ML, Price FG. A Case-Series Observation of Sweat Rate Variability in Endurance-Trained Athletes. Nutrients. 2021; 13(6):1807. https://doi.org/10.3390/nu13061807
Chicago/Turabian StyleSmith, JohnEric W., Marissa L. Bello, and Ffion G. Price. 2021. "A Case-Series Observation of Sweat Rate Variability in Endurance-Trained Athletes" Nutrients 13, no. 6: 1807. https://doi.org/10.3390/nu13061807
APA StyleSmith, J. W., Bello, M. L., & Price, F. G. (2021). A Case-Series Observation of Sweat Rate Variability in Endurance-Trained Athletes. Nutrients, 13(6), 1807. https://doi.org/10.3390/nu13061807






